Design and Development of Fenofibrate Solid for Solubility Enhancement
DOI:
https://doi.org/10.5530/ctbp.2023.1.8Keywords:
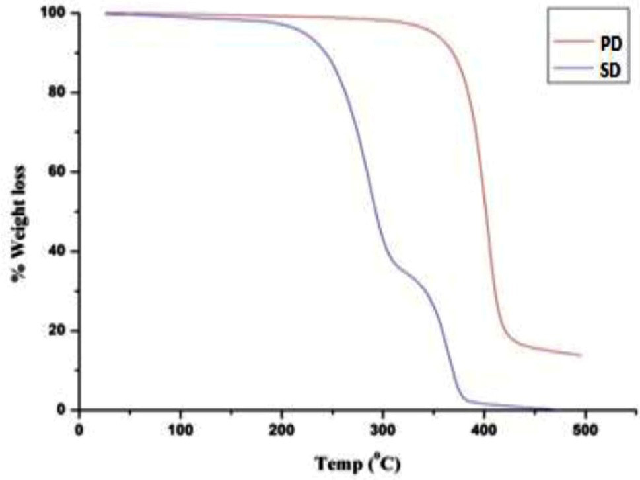
Design, Development, Fenofibrate, Solid Dispersions, Solubility, Hydroxyl propyl methyl cellulose.Abstract
This research work is designed to over-come the problems associated with the poorly aqueous soluble drug. Fenofibrate (FENO) be-ing a BCS Class II drug shows low solubility with high permeability. Compatibility of the drug and polymer studies was done by FTIR. Physical mixtures (PM) of the drug and polymer HPM-CAS were prepared by the trituration method. Fenofibrate solid dispersions (SD) were pre-pared by a common solvent technique ascribed to feasibility on a laboratory scale. The physi-cal state of formulations was characterized by powder XRD and TGA. Solubility of a pure drug (FENO), Physical mixture, and SD were found to be 0.3, 0.78±0.15 to 1.15±0.28, and 2.17±0.37 to 3.25±0.14 mg/ml respectively. Percentage yield and percentage drug content were deter-mined and found within a satisfactory range. The maximum cumulative percentage of drug release from the pure drug (FENO), Physical mixture, and SD was found to be 34.5%, 72.1 and 97.2% respectively at 60 minutes. A micros-copy (SEM) study found that the prepared solid dispersion has porous morphology. The present study establishes the increased bioavailabili-ty of the optimized batch when compared with the pure FENO. There was a significant (50%) increase in absorption of Fenofibrate observed from the in vitro everted gut sac model. SD of FENO was developed successfully. The solubil-ity of FENO was ameliorated significantly while compared with API (pure FENO). This research work found the formulation of SD preferable technique to enhance solubility and enhance dissolution of lipophilic drugs