Preparation and characterization of oral fast dissolving film of hydralazine HCL
DOI:
https://doi.org/10.5530/ctbp.2022.3s.75Keywords:
Hydralazine Hcl, Oral fast dissolving films, Hydroxypropyl methylcellulose, Sodium carboxy methyl cellulose, Solvent casting method.Abstract
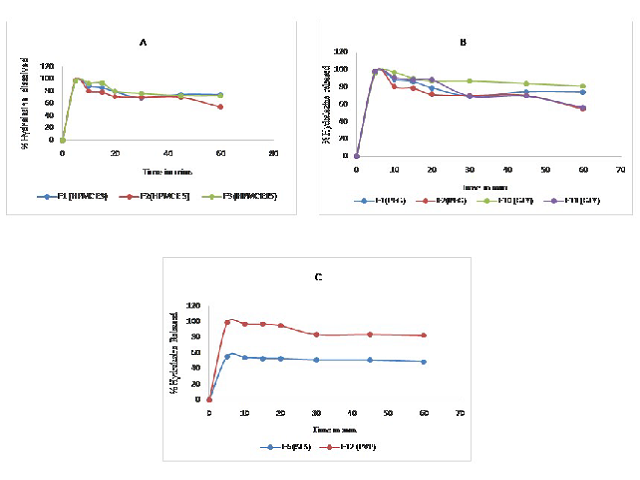
Hydralazineis a BCS class III antihypertensive drug. The main aim of this study is to improve the dissolution and thus the bio availability of hydralazine by preparing fast dissolving oral films of Hydralazine HCl. The objective of this study is to enhance therapeutic efficacy, compliance & convenience of geriatric and pediatric patients by preparing hydralazine fast dissolving oral films by solvent casting method. The hydralazine films were prepared by using different film forming polymers. Various grades and concentrations of hydroxypropyl methylcellulose (HPMC) E 3 and E 15, methyl cellulose (MC), sodium carboxy methyl cellulose (CMC), varying the surfactants like Sodium Lauryl Sulfate (SLS) and Poly vinyl pyrrolidone (PVP) are used. Morphological studies, Thickness uniformity, Folding endurance, Drug content uniformity test, In vitro dissolution studies, Weight variation tests were performed for evaluation of films.The film formulation F12 having HPMC E 15, PVP and Glycerol showing the greatest dissolution and bio availability and could give quick onset of action upon administration when compared to other formulations.