Development and Validation of a Sensitive and Rapid Bioanalytical RP-HPLC Method for the Quantification of Nebivolol Hydrochloride in Rat Plasma
DOI:
https://doi.org/10.5530/ctbp.2022.3s.67Keywords:
Bioanalytical, Reversed Phase HPLC, Nebivolol HCl, ICHAbstract
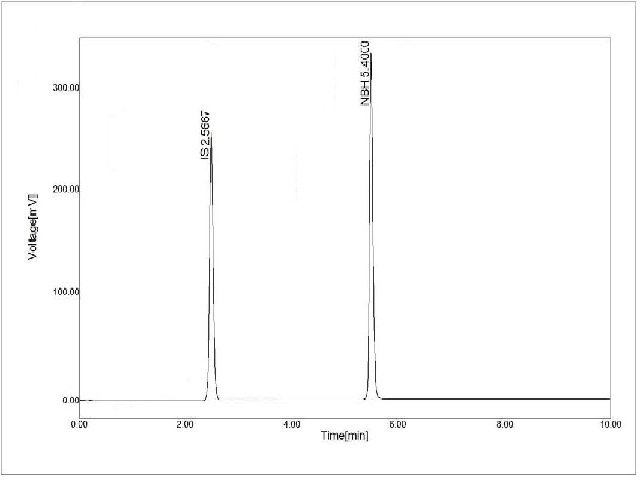
A simple and specific bioanalytical reverse phase high performance liquid chromatography (HPLC) assay method was established and validated for estimation nebivolol HCl in rat plasma using amlodipine besylate as internal standard. The chromatographic separation was achieved by reversed phase Knauer C18 column and mobile phase composition of acetonitrile, methanol and 0.1N orthophosphoric acid (80:20:10) with ultraviolet detector at the wavelength of 272 nm. The flow rate was maintained at 0.5 mL/min. System suitability parameters of mobile phase composition, flow rate, and wavelength were optimized to provide highly sensitive peaks for the drug samples. The calibration curve was found linear in the range of 400 ng/mL to 1800 ng/mL with a retention time of 5.48 minutes. LOD and LOQ were found at 40.24 ng/mL and 121.94 ng/mL respectively. The developed method was validated in terms of linearity, recovery, precision, robustness, ruggedness, and stability (short term & longterm stabilities and freeze & thaw stability). All the experiments were done as per ICH guidelines and the outcomes were well inside the limits determined.