RP-HPLC Method for Determination of Favipiravir (RdRp of RNA Viruses) in Pharmaceutical Dosage Form
DOI:
https://doi.org/10.5530/ctbp.2022.3s.63Keywords:
RP - HPLC, Favipiravir, Validation, ICH guidelines.Abstract
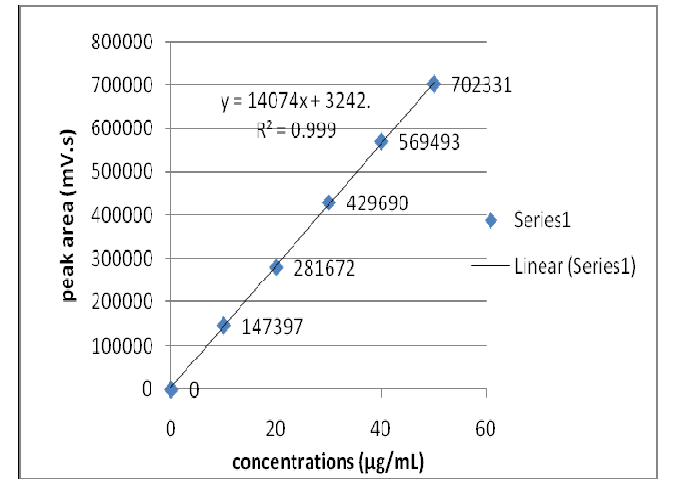
A simple, sensitive, precise, accurate, rapid, and reproducible HPLC method was developed and validated for the determination of Favipiravir (RdRp of RNA viruses) in pharmaceutical dosage form. Good quality chromatographic separation of Favipiravir (FAV) was conceded out by using Nucleosil C18 column (4.6 mm i.d., X 250 mm., 5 μm particle size) (based on 99.999 % ultra-high purity silica) using mobile phase consisting acetonitrile: methanol: HPLC Grade water (50:40:10 % v/v) at flow rate of 1 ml/minute. The λmax of the Favipiravir was found to be 365 nm. The retention time of Favipiravir was found to be 2.794 min and the calibration curve was linear function of drug in the conc. range of 10-50 μg/mL (r2 = 0.9998). The LOD and LOQ were found to be 1.042995 μg/mL and 3.160591 μg/mL respectively. The recovery (accuracy) studies were performed and the percentage recovery was found to be 99.34 - 99.42 %. Percentage assay of Favipiravir tablets were found to be 99.85 %. Infact the % RSD values for all validation key parameters were less than 2 %. Thus, the developed method found to be fruitfully practicable for the determination of Favipiravir in quality control analysis in pharmaceutical formulations.