Development and evaluation of capecitabine loaded human serum albumin nanoparticles for breast cancer
DOI:
https://doi.org/10.5530/ctbp.2022.3s.61Keywords:
Capecitabine, Human Serum Albumin, Desolvation method.Abstract
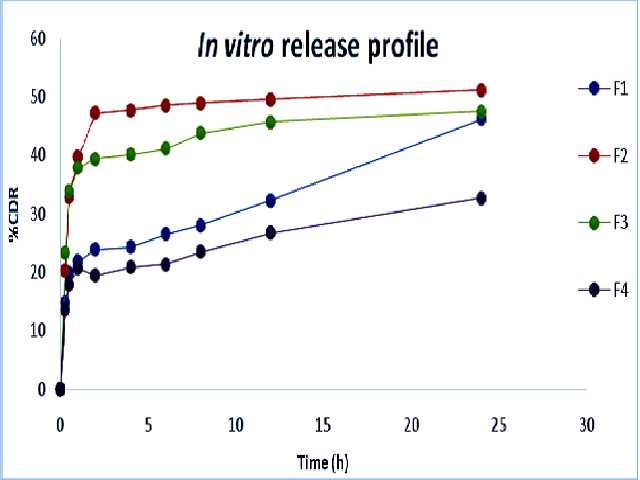
Capecitabine is an orally-administered chemotherapeutic agent used in the treatment of metastatic breast and colorectal cancers. Albumin nanoparticles indicated high drug loading capacity in a composite with biodegradability and biocompatibility. The aim of the present study is to develop capecitabine loaded albumin nanoparticles and their evaluation. Capecitabine loaded albumin nanoparticles were prepared by desolvation method. The prepared nanoparticles were characterised for mean particle size, zeta potential, and drug loading capacity. The process yields and drug loading ranged between 66%w/w to 84.6%w/w and 5.06%w/w to 15.36%w/w respectively. The mean particle size of nanoparticle size of various batches F1 to F4 were found to be 151.18nm, 134.9nm, 178.8nm, and 151.3nm respectively. The zeta potential of the batch F2 was found to be - 21.1mV. The drug release was ranged from 32.75% to 51.2% for 24h depending on drug polymer ratio. The in vitro release studies showed a biphasic release pattern with an initial burst effect followed by sustained release. Release kinetic model revealed that the mechanism of drug release from human serum albumin nanoparticles followed Fickian model.