Putative Molecular Mechanisms of Neuroprotective Cerebrosides and their Docking studies on Acetyl Cholinesterase Enzyme Inhibition for the Treatment of Alzheimer’s disease
DOI:
https://doi.org/10.5530/ctbp.2023.4.78Keywords:
Eukaryotes, cerebrosides, neuroprotection, Alzheimer’s disease, molecular dockingAbstract
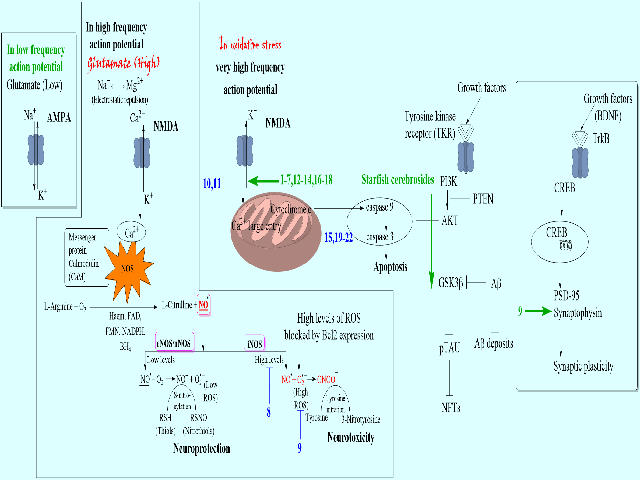
The Dementia disease is characterised by neuropsychiatric disturbances due to lack of proper synaptic communication between neu- rons causing the cognitive behavioural prob- lems. The Alzheimer’s disease (AD) in elderly population is one of the several forms of De- mentia. Recent data by World Health Organisa- tion indicates that nearly 10 million people are getting dementia every year, of which 60-70% accounts for AD. The etiology of AD involves the formation of amyloid-β plaques and neu- rofibrillary Tau tangles in the brain resulting in the death of neural cells. There is no perma- nent solution for AD treatment, except the FDA approved drugs like galantamine, donepezil, rivastigmine and memantine that are normally associated with side effects. At this juncture, ce- rebrosides, the natural secondary metabolites identified from different taxa with potential neu- roprotective effects offer a promising scope for the treatment of AD. In this paper, cerebrosides reported from fungi, plant and animal taxa are pooled up along with their functions and listed. The review of literature revealed that Cerebro- sides can increase the cognitive functions by regulating or interacting with the N-methyl-D-as- partate (NMDA) calcium ion (Ca2+) channels at post-synaptic receptor; nitric oxide (NO); Bcl2, Bax, amyloid precursor (APP) and Tau pro- teins; brain-derived neurotrophic factor (BDNF) and cAMP- response element-binding proteins (CREB). This indicates that the Cerebrosides could be potential therapeutic agents for the protection of neurons involved in neurodegen- erative disease like Alzheimer’s disease. The current neuroprotective drugs are AChE inhibi- tors; hence, in the present investigation, in silico molecular docking study on cerebrosides for the inhibition of AChE was assessed to find out their capacity to interact with an active catalytic site of AChE. The results of present investigation re- vealed that all 22 cerebrosides selected for this work interacted with catalytic active site of AChE measured in terms of Gibbs free binding energy. Of all the cerebrosides assessed, compound 6 exhibited strong interaction, followed by 15. This is the first report of molecular docking study on cerebrosides for AChE enzyme inhibition for treatment of Alzheimer’s disease. Nevertheless, detailed in vitro and in vivo, biochemical and molecular investigations are needed to bring them to useful form