Substrate Specificity of Paraben Towards Liver Esterase: An In-Silico and Titrimetric Analysis
DOI:
https://doi.org/10.5530/ctbp.2021.6.20Keywords:
Paraben, Preservative, Docking, Esterase, Binding energy.Abstract
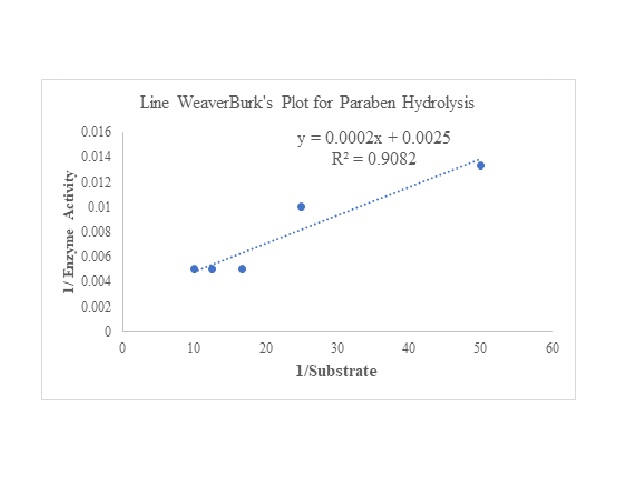
Parabens, esters of para-hydroxybenzoic acid, have so far been “safely” used in our daily life as a preservative in most of the commercial products including cosmetics. It is the relatively inert, non-reactive, odorless nature and easy miscibility with the majority of the products that make it a suitable choice as preservative. However, recent studies have explored the dark side of the uses of this compound, as a potential environmental hazard due to its impact on humans as endocrine disruptor and carcinogen. In current study in-silico interactions between one such most extensively used Paraben, methyl Paraben (MP) and Porcine liver esterase were explored. Based on docking studies and binding energies of substrate (ethyl butyrate) and MP, it can be proposed that Porcine liver esterase can be a potential enzyme for MP hydrolysis. Also, the titrimetric method for esterase assay was also performed. The kinetic parameters, analysed using Line WeaverBurk’s plot showed Km and Vmax to be 0.08 mM and 400 U/ml, respectively. The studies proposed that MP can be a potential substrate for hydrolysis by Porcine liver esterase, at pH 8.0 and 25ºC. The study will open the avenue of using enzymes for designing analytical tools for detection of Paraben.