A Review on COVID-19 and Vaccine Development: What We Have Learned in this Pandemic
DOI:
https://doi.org/10.5530/ctbp.2025.4s.20Keywords:
COVID-19, vaccines, coronavirus, SARS-CoV-2 virus, immunityAbstract
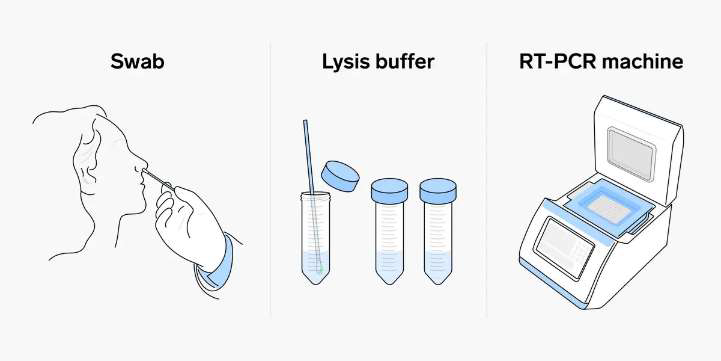
The emergence of the Coronavirus disease (COVID-19) was initially documented in December 2019 in Wuhan, China which quickly evolving into a global pandemic. COVID-19 is caused by the Severe Acute Respiratory Syndrome coronavirus 2 (SARSCoV- 2) virus. While the majority of individuals infected experience mild to moderate respiratory symptoms and recover without specific treatment, a minority endure severe illness requiring intensive medical care.In response, scientific/academic institutions and pharmaceutical manufacturers embarked on the development of COVID-19 vaccines, rigorously gathering evidence on their safety and efficacy. Regulatory authorities meticulously assess this data prior to international distribution. This article discusses on the life cycle and host cell invasion mechanisms of SARS-CoV-2 virus, pathophysiology of COVID-19, diagnostic tests, preventive measures, and vaccines development. Overall, this review offers a thorough comprehension of the COVID-19 pandemic, providing stakeholders with the knowledge needed to effectively respond to and mitigate its impact on public health and society.