Synthesis and Characterization of Capsule Shell Formulation Using Konjac Glucomannan as an Alternative Halal Binder
DOI:
https://doi.org/10.5530/ctbp.2025.4s.12Keywords:
Konjac Glucomannan, capsule, alternative, halal binderAbstract
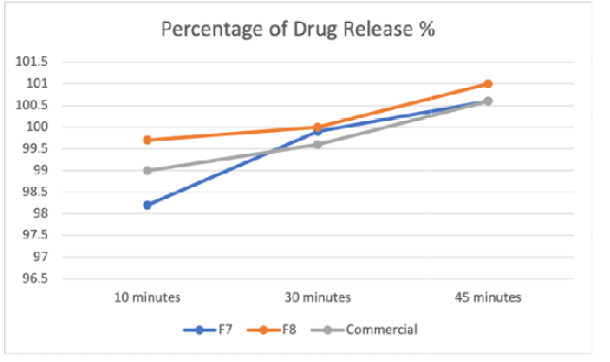
Background: In accordance with cultural and religious restrictions, certain consumers, notably among Muslims, Hindus, Jews, and vegetarians around the world, are concerned when gelatin used in capsules is produced from animal sources, particularly porcine and bovine. Therefore, an effort to develop halal, plant-based pharmaceutical ingredients and excipients has been made to solve this issue. Less studies have been conducted on Konjac Glucomannan in capsule production. Objective: This research was conducted by developing alternative plant-based capsule through dipping method and find the best formula that can achieve and fulfill good properties of capsules according to standards. Methods: Hard capsule shells were made using formulations such as konjac powder, sorbitol, starch, lactose and distilled water using dipping method. The best formulation of capsules was selected and then they were tested using weight variation test, swelling degree test, water content analysis, disintegration test and dissolution test to evaluate their physicochemical properties. Results: Based on the results of the study, antimicrobial of synthetic konjac powder is very minimal and to get optimum results of antimicrobial, extraction of Konjac Glucomannan from its plant is needed. There were nine formulations produced, however only three of the formulations were chosen which were formulation F7, F8 and F9. Formulation F8 was selected as the best formulation as its characterization results fulfils the standard requirement. Conclusion: In a nutshell, the usage of Konjac Glucomannan as an alternative binder is appropriate in production of capsules with addition of other polymers such as starch and lactose.