HPLC Method Development and Validation for Simultaneous Estimation of Dapagliflozin and Linagliptin in Bulk Drug and Pharmaceutical Formulation
DOI:
https://doi.org/10.5530/ctbp.2025.4s.8Keywords:
HPLC, Dapagliflozin, Linagliptin, Diabetes Mellitus, Pharmaceutical Analysis, Method ValidationAbstract
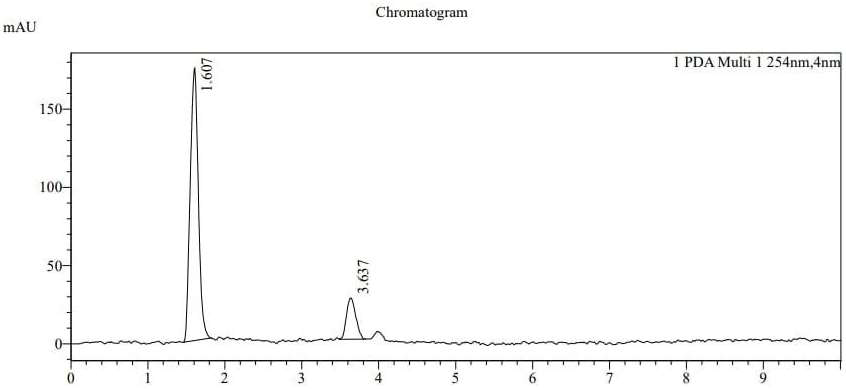
Diabetes mellitus is a chronic metabolic disorder characterized by persistent hyperglycemia, often leading to severe complications such as cardiovascular disease, renal failure, and neuropathy. Linagliptin, a DPP-4 inhibitor, and dapagliflozin, an SGLT2 inhibitor, are frequently used together to treat type 2 diabetes mellitus in order to enhance glycemic control. The objective of this research is to develop and validate a novel new high-performance liquid chromatography (HPLC) technique for the simultaneous detection of linagliptin and dapagliflozin in pharmaceutical dosage forms and bulk. The chromatographic separation was carried out using a Waters Reliant C18 column (150 × 4.6 mm) with a mobile phase consisting of acetonitrile and water (60:40 v/v) in isocratic mode. The flow rate was maintained at 1.0 mL/min, with UV detection at 244 nm. The retention times for Dapagliflozin and Linagliptin were found to be 1.607 and 3.637 minutes, respectively. The method was validated according to ICH guidelines for accuracy, precision, linearity, robustness, and ruggedness. Linearity was established in the concentration range of 60- 210 ppm for Dapagliflozin and 1-10 ppm for Linagliptin, with correlation coefficients (r²) above 0.998. Recovery studies confirmed accuracy within acceptable limits. The developed method proved to be precise, accurate, and robust, making it suitable for routine quality control analysis of Dapagliflozin and Linagliptin in pharmaceutical formulations. This validated method ensures reliable quantification, facilitating effective therapeutic monitoring of these antidiabetic agents.