Formulation Development and Characterization of Ritonavir Loaded Controlled Release Matrix Tablet
DOI:
https://doi.org/10.5530/ctbp.2025.2.16Keywords:
Ritonavir, Matrix tablet, Hydroxy- propyl methyl cellulose, Controlled release tablet, Human immunodeficiency virus proteaseAbstract
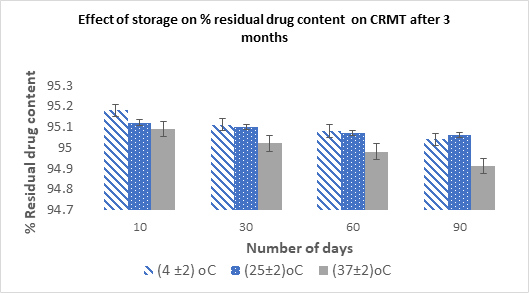
The aim of this study was to design Ritonavir loaded controlled-release matrix tablet (CRMT) for the treatment against Human immunodeficiency virus, with an emphasis on the drug’s pharmacokinetic and physicochemical characteristics for enhanced therapeutic efficacy and decreased gastrointestinal side effects. The tablet was prepared by using rate-controlling polymers like Hydroxy-propyl methyl cellulose (HPMC) K4M and xanthan gum by direct compression method. Formulation batches F1-F9 were developed, optimized using 32 full factorial design and evaluated for pre and post compressional studies. Drug-excipients studies were performed by IR spectra and DSC thermogram. All batches of powder blends were evaluated for particle size, angle of repose, bulk density, tapped density, % Compressibility index and Hausner’s ratio. The prepared tablets were characterized for Weight variation, Hardness, Thickness, Friability, % Drug content, % Swelling index and In-vitro cumulative drug release study up to12h. Analysis of variance was used to handle acquired data for statistical analysis. In conclusion, maximum drug release 96.29% and swelling index 81.29±0.09%, was observed in F9, after 12 h of studies and found stable under short term stability study as per ICH guideline. The article developed a potential scope in reducing the dose-dependent gastrointestinal toxicity of ritonavir with fewer side effect and a hope in future.