Engineering Cefpodoxime Prodrug using Nanosuspension Approach to Modulate Solubility, Antimicrobial and Pharmacokinetic Profile
DOI:
https://doi.org/10.5530/ctbp.2025.2.13Keywords:
Cefpodoxime proxetil, Nanosuspension, Prodrug, Solubility enhancement, Oral bioavailability enhancement, Pharmacokinetic studyAbstract
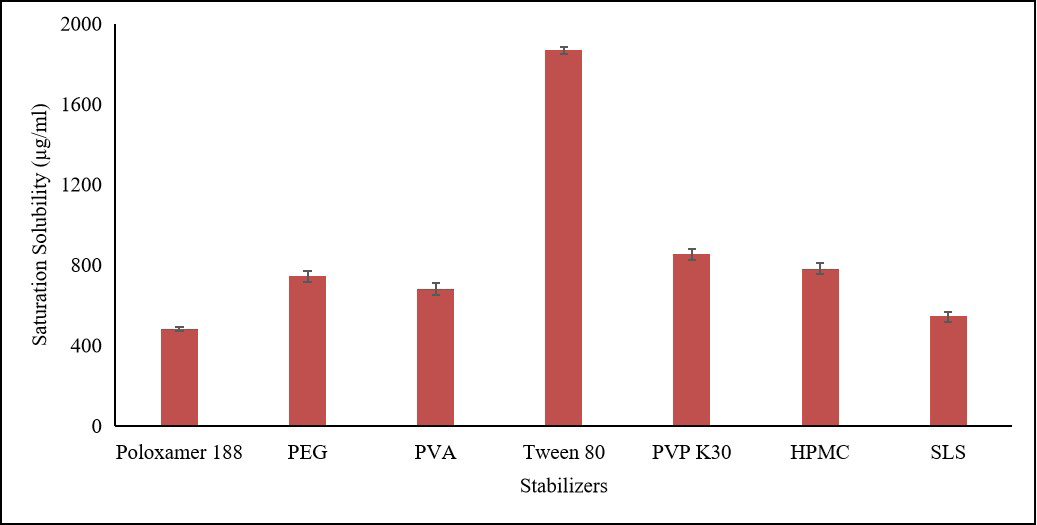
Cefpodoxime proxetil (CP) is broad-spectrum antibiotic belongs to third-generation cephalosporin family. Its low solubility and bioavailability have been a challenge for drug delivery. Nanosuspension (NS) technology has been explored in drug delivery to address the issues of drugs with poor water solubility. The study focused on developing a CP nanosuspension (CP-NS) formulation using solvent-antisolvent precipitation technique. The CP-NS was synthesized by precipitation using 0.5 % w/v sodium lauryl sulphate and 1.5 % w/v poloxamer-188 under controlled ultrasonication. CP-NS was characterized for Fourier transform infrared (FTIR), Transmission electron microscopy (TEM), Differential scanning calorimetry (DSC), and X-ray diffraction (XRD). In vitro dissolution studies revealed that CP-NS exhibit increased dissolution rate 2-folds than pure drug and 1.3-folds higher than marketed formulation. In vivo pharmacokinetic studies revealed 4.3- fold improvement in oral bioavailability of CPNS than pure drug and marketed formulation. In conclusion, the formulation of cefpodoxime proxetil nanosuspension showed promising results in terms of drug dissolution and antimicrobial activity for prodrug based active moieties.