Method development and estimation of phenylenediamine in gastric contents, blood and urine
DOI:
https://doi.org/10.5530/ctbp.2021.1.3Keywords:
Phenylenediamine, Pharmaceutical Science, Method developmentAbstract
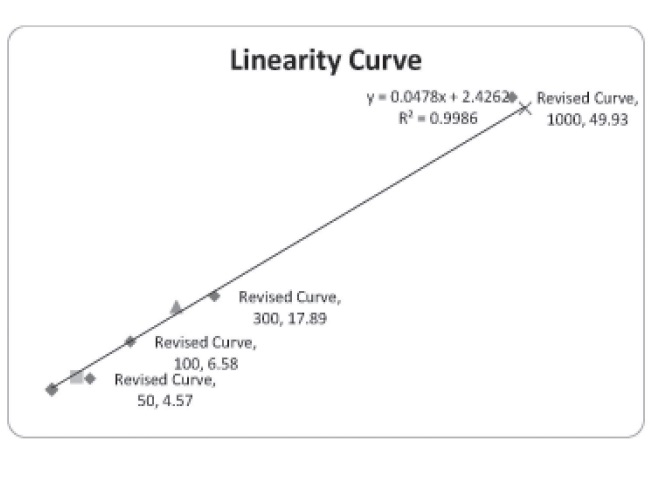
PhenylenediamineA method for detecting and estimating Paraphenylenediamine (PPD) in the biological samples has been developed and validated using High- Performance Liquid Chromatography (HPLC) with UV/VIS detector. The method quantitatively measured the PPD in the biological samples. The mobile phase was composed of 10: 90 (Acetonitrile: Ammonium acetate 25 Mm v/v) of pH 4.5. The isocratic separation was carried out at C18 silica gel column at 1ml/minute of flow rate. The spectrophotometric detection was carried out at 240 nm. The process was validated, and the limit of detection for PPD was found to be 10μg. The accuracy and precision of the process were within limits. The linearity of the process found to be upto 1000 ppm. The extraction of PPD was carried out with chloroform and Sodium Hydroxide. The stock solution of PPD was freshly prepared due to oxidation potential. Aniline was used as an internal standard. The sample negative and sample positive were run to ensure the validity of the process. The estimation of the PPD was performed by drawing the calibration curve of different calibrators of PPD. The determination of PPD was carried out in gastric contents, Blood, and Urine. The extracted samples were prepared and run at HPLC for the detection. The pretreatment of the samples was not required, and the method proved to be accurate, precise, and specific.