A Qualitative Study on the Implementation of Sertu in Pharmaceutical Industry: Processes, Issues and Challenges
DOI:
https://doi.org/10.5530/ctbp.2024.3s.8Keywords:
Halal pharmaceuticals, Halal medicines, Seru cleansing, Halal pharmaceutical industry, Good Manufacturing Practice(GMP)Abstract
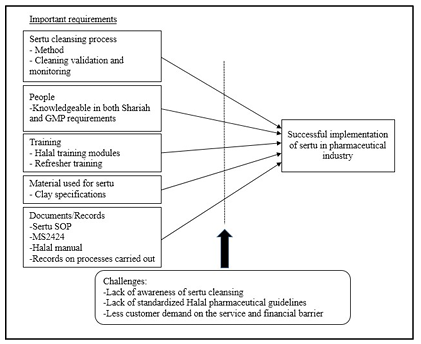
Recently, halal pharmaceutical is starting to get the place in the market and is well-accepted among Muslim and non Muslim consumers. To be certified halal by JAKIM, emphasis is notonly on the origin of the ingredients, but also the manufacturing process whether it complieswith the requirements set by halal pharmaceutical standards. According to Malaysian Standard, MS2424 Halal Pharmaceuticals Guideline, sertu cleansing need to be conducted if contamination with severe najs (naj mughallazah) materials occurs. However, there have been very few studies conducted on halalpharmaceuticals especially on sertu cleansing in pharmaceutical industry. Hence, this exploratory qualitative study was conducted to provide insight into the way sertu process is carried out in the pharmaceutical industry, and to determine the importantenabling factors needed and the challenges faced in an effort to establish a halal-certified pharmaceutical industry. The data was collected through semi-structured in-depth interviews in two halal-certified pharmaceutical companies in Malaysia. The study pharmaceutical industry and the challenges faced by industry players along the sertu implementation process which inludes lack of awareness of sertu cleansing by the halal certified pharmaceutical industry players and Muslim consumers, issues with the standard and financial issues in implementing the sertu. A proposed conceptual framework was developed from the findings of the study.