A Systematic Literature Review on Alternative Options For Halal Critical Ingredients In Halal Pharmaceutical and Cosmetics
DOI:
https://doi.org/10.5530/ctbp.2024.3s.3Keywords:
halal, critical ingredients, systematic literature review, insulin, gelatineAbstract
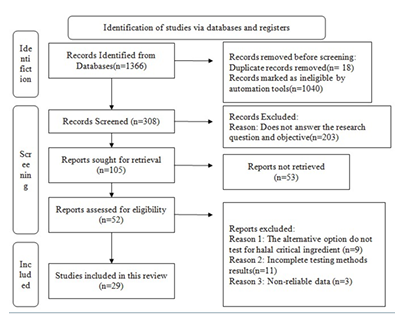
The global halal market is expanding fast as halal products establish themselves as a new standard for safety and quality assurance. The constituent of a product, whether pharmaceutical or cosmetic, determines its halal classification. Ingredients that do not correspond to the halal standard are commonly known as critical ingredients. As a result, various substitutes for critical ingredients should be developed to raise global demand for the halal market. This study aims to review the current research development on the alternatives for halal critical ingredients in halal pharmaceuticals and cosmetics and to explore the testing methods used to test the alternative option for halal critical ingredients. This systematic study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards and all the publications in this review meet the research eligibility requirements, which were searched and selected using electronic databases such as PubMed, Scopus, and MyCite. This study examined approximately 21 publications that proposed various substances derived from sources such as plants, animals, marines, and microbes. Three publications illustrated the possible use of the choice in insulin resistant patients, whereas seven articles discussed on the potential replacement for gelatine. Insulin and gelatine are the most frequently explored topics among the publications included in this review. Their alternative possibilities, whether derived from plants, marine sources, or microbe based substances, are thoroughly evaluated to determine their desired effect or activity. The testing methodologies demonstrated that the alternative possibilities are far superior to the critical ingredients in terms of texture, morphology, activity, composition, and even the cost of synthesis.