Transcriptional and translational leakiness in expression of a fungal cyclopropane fatty acid synthase in the Escherichia coli pET-vector expression system
DOI:
https://doi.org/10.5530/ctbp.2021.1.1Keywords:
pET-vector, heterologous protein expression, basal transcription, translational leakiness, Shine Dalgarno (SD) sequenceAbstract
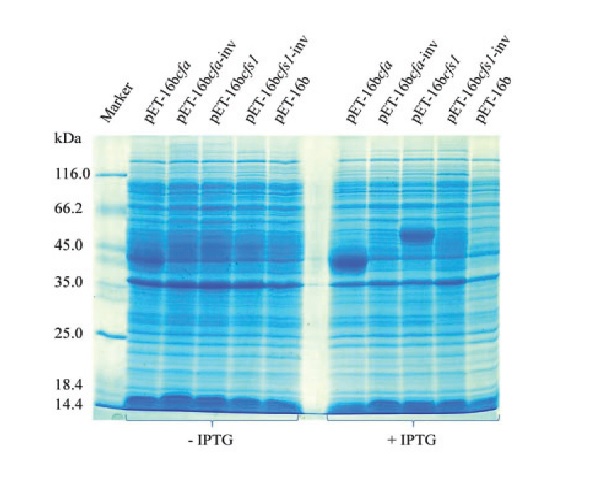
We expressed the putative cyclopropane synthase Cfs1 from the mushroom Coprinopsis cinerea and the native bacterial Cfa enzyme in Escherichia coli as fusion proteins with an Nterminal 10xHis-tag and a proteolytic Factor Xa site, using the commercial pET-16b vector with the T7lac promoter for expression. Proteomics detected both proteins with high Mascot scores via trypsin-digested peptides in total cytoplasmic protein samples from transformed BL21(DE3) bacteria, induced by IPTG (isopropyl β-D-1- thiogalactopyranoside) for T7 RNA polymerase production from theT7 phage gene 1 present in the host genome under control by the lacUV5 promoter. In lower amounts, Cfs1 and Cfa were also found in protein samples from non-induced cells based on the known transcriptional leakiness of the pET-system in expression of T7 RNA polymerase. As a novelty, the results on Cfs1 from proteomics suggest that the sequence at the pET-16b-cfs1 fusion point CTCGAGGAT CCTATG (BamHI cloning site in italic, native cfs1 ATG start codon in bold) serves in transcripts as a minor RBS (ribosome binding site) to inadvertently initiate protein synthesis on the native cfs1 start codon. The first peptide MPAHHHPSSSAPCVSFPSSSK of the native Cfs1 protein was found in E. coli which is only possible if the normal cfs1 ATG was recognized as the start codon in protein biosynthesis. The GAGG-stretch in the above DNA sequence with a 7-bases-aligned spacing to the native cfs1 start codon features a partial Shine-Dalgarno (SD) sequence.