Development and Validation of a RP-HPLC Method for the Determination of Capecitabine and its Impurities in Pharmaceutical Dosage Form
DOI:
https://doi.org/10.5530/ctbp.2024.1.3Keywords:
Capecitabine, Assessment of related substances, LOQ, Forced Degradation and Liquid chromatographyAbstract
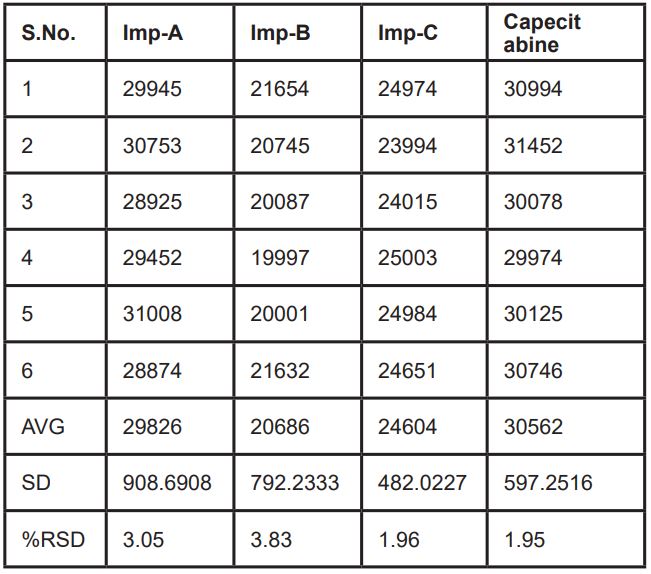
The analysis of established HPLC technique for the separation and quantification of Capecitabine and its impurities are described. Samples are analysed by reverse phase (RPHPLC) using stationary phase Inertsil ODS-3V (250 x 4.6mm, 5µm) column and the movable segment consisted of two channels. Channel A: 20mM Ammonium Formate buffer, Methanol and Acetonitrile in the proportion of (75:25:5 %volume/volume/volume) and Channel B: 20mM methanol, ammonium acetate buffer and Acetonitrile in the proportion of (80:15:5 %volume/volume/volume). The run velocity is 1.0 mL/min, the column oven was preserved at 40°C and sampler cooler oven was preserved 5°C, infused 10µL and wavelength fixed at 250nm UV-detection. The established HPLC technique was authenticated with admiration to specificity, precision, linearity, accuracy, LOD, LOQ and solution stability. Corroboration study compared as stated by ICH instruction.