Exploring the potential of Designed Multiple Ligands (DML) strategy with quinolones as anticancer
DOI:
https://doi.org/10.5530/ctbp.2023.4s.84Keywords:
quinolones, anticancer, topoisomerase inhibitor, tyrosine kinases, DML, hybridsAbstract
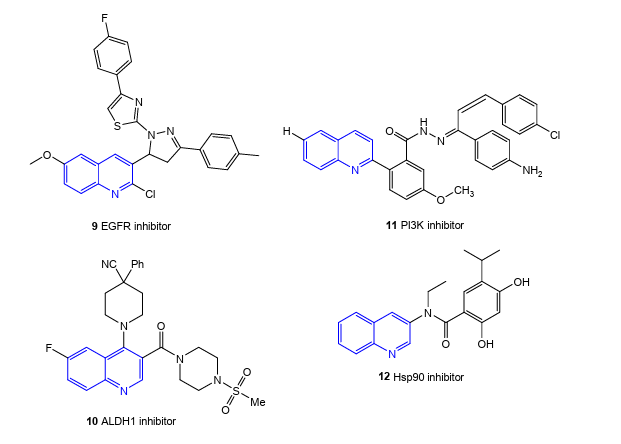
Multi-target-directed ligands (MTDL) or Designed Multiple Ligands (DML) use a single chemical substance to affect several ligands or targets associated with a disease to boost efficacy or safety. In recent studies, many novel quinolones have adapted this strategy by targeting many cancer ligands, including topoisomerase, tyrosine kinase, tubulin polymerisation, and formation of Gquadruplex. Moreover, the effectiveness of anticancer quinolones has been improved by the conjugation of compounds with metal complexes, such as ruthenium (III), boron, and copper (II). In the case of dual inhibitors, most of the substances target topoisomerases along with additional targets such as histone deacetylases, telomerase, microtubules, kinases, heat shock protein 90 (Hsp90), aldehyde dehydrogenase 1 (ALDH1) and proteasomes. Some of these hybrids, such as CX-5461, Q84441, and A-74441, have been shown to be effective against solid tumors with improved safety profiles. In this review, the current quinolone hybrids and DML strategy against a range of targets will be examined with the hope that the insights will aid in the development of novel quinolone derivatives for cancer treatment.